Project A3 - Excitation Transfer
Non-equilibrium excitation of CO2 in an atmospheric pressure helium plasma jet
First results on the non-equilibrium excitation and dissociation of CO2 in an atmospheric pressure helium RF plasma jet. The objective of the project A3 in the SFB 1316 and the BMBF project Carbon2Chem is the separation of plasma and surface chemistry studied for the example of CO2 plasma excitation admixed to the noble gas within the plasma jet. This method offers the possibility to control the gas temperature of the feed gas as well as the molecule excitation by low energy electrons or by Penning collisions with the excited noble gas atoms or dimers. The plasma jet is driven with varying absorbed plasma power and admixture levels of CO2 . The excitation of CO2 is monitored by in-situ set-up of Fourier-Transform Infrared Spectroscopy. Concetrations of CO2 and the produced CO are analysed. Furthermore, the vibrational and rotational temperatures of the possible degrees of freedom of the measured molecules are determined.
The gas feed of the atmospheric pressure plasma jet was helium, because of the large mass difference and, there- fore, poor momentum transfer to CO2. This results in the smallest collisional quenching of all noble gases. The plane parallel plasma jet is driven with RF. These kind of plasma source is already very well investigated in respect to their plasma physics and chemistry for different gas mixtures of noble gases and molecules.
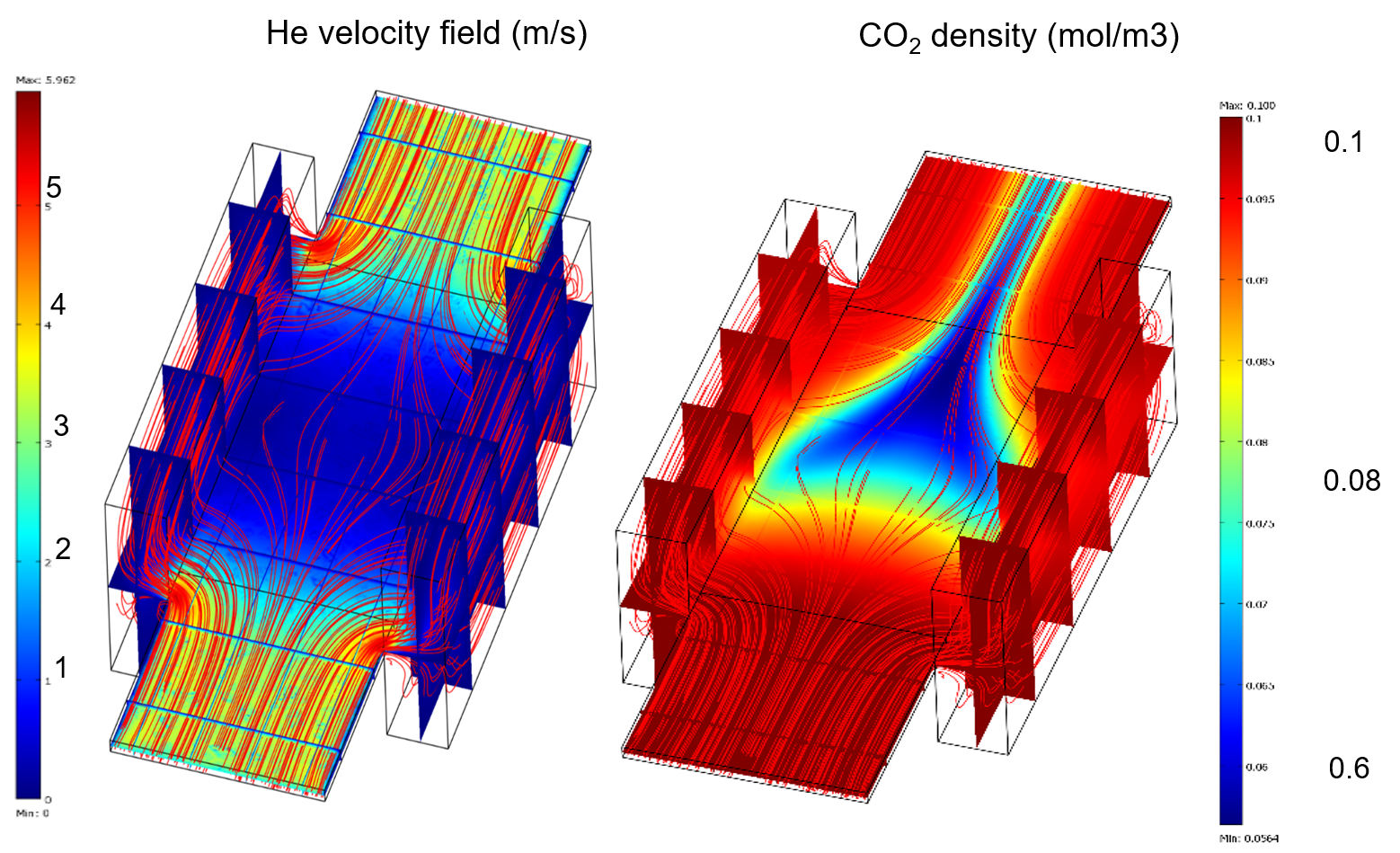
The main result of this work is the clear non-equilibrium excitation of CO2 and CO. In detail, the rotational tem- perature of CO is below 400 K and, in contrast to this, the vibrational temperature reached values up to 1600 K, and the temperature of the excitation of the asymmetric vibration of CO2 is about 700 K. The impact of variable plasma power and admixture of CO2 to the He gas flow is rather weak. It is assumed that the vibrational and rotational excitation of CO mainly originates from the dis- sociation reaction either by direct electron impact of CO2 or by Penning dissociation between CO2 and excited helium metastables. From this electronic energy transfer to CO2, highly vibrational excited CO molecules are pro duced by dissociation.
The non-equilibrium is due to the nature of excitation of molecules by collisions with electrons with energies larger than 7 eV at the oscillating sheath edges and Penning collisions with excited heilum atoms. The low rotational gas temperature is explained by the helium plasma gas which acts as a buffer.
This non-equilibrium character offers more investigations within the field of plasma catalysis, through which the reaction rate of a desired catalytic reaction is supported. Moreover, it is important to ensure the en- hancement of the reaction rate through the impact of excited molecules and not due to an unintentional heating of the catalyst surface by the plasma itself.
In the future, the experiments will be extended to other gas mixtures and the impact of catalytically active surfaces will be explored.

