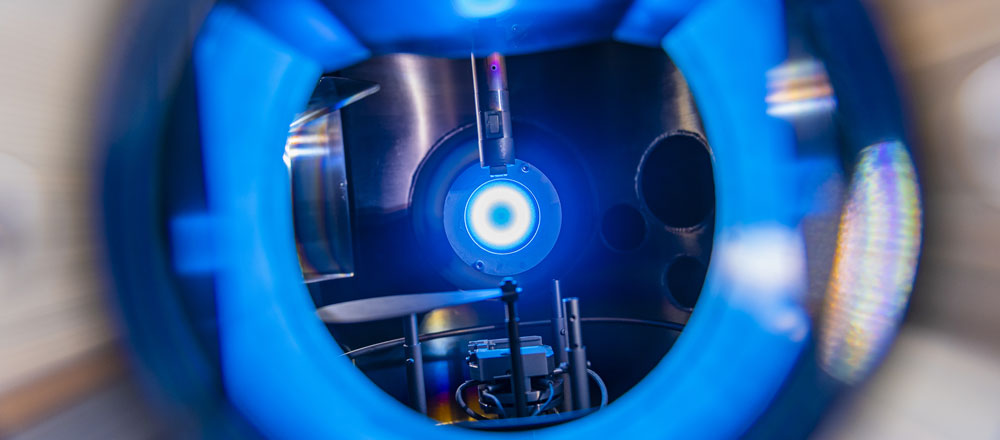
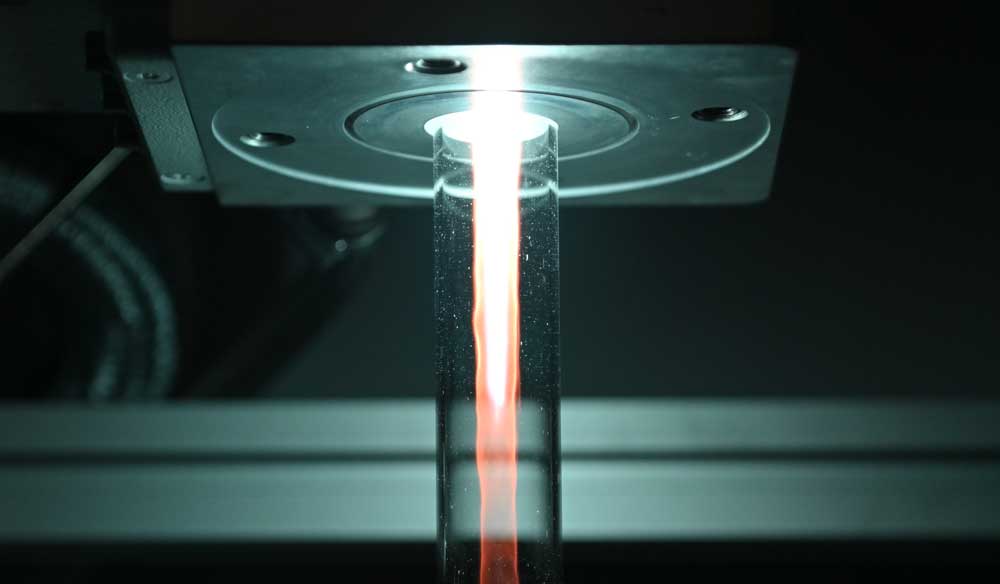
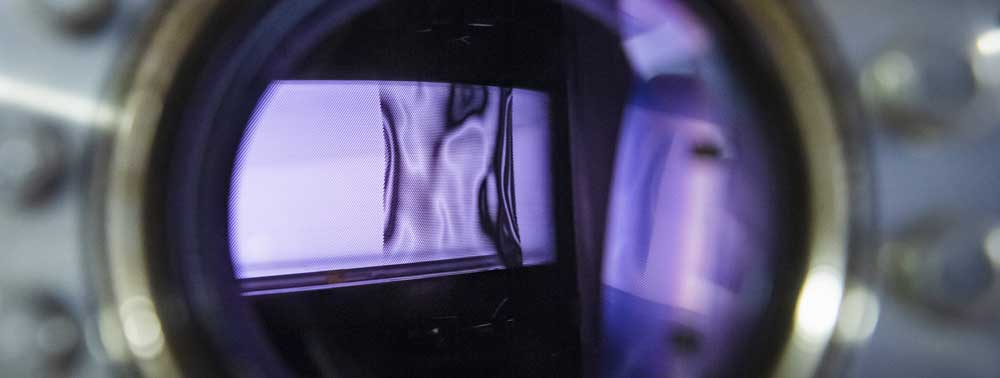
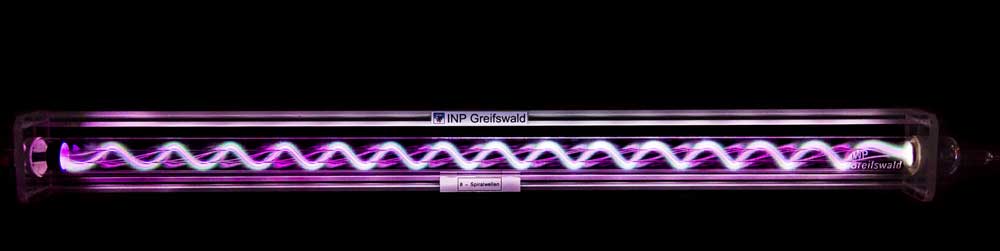
A3,A7 - Plasma ChemistryGas Conversion in an sDBDSurface Dielectric barrier discharges are perfect |
- Details
Working group plasma electrolysis
The Future German and European Energy System – The Role of Hydrogen and its Production, Transport, and Use
A plasma workshop will take place with a special lecture on electrolysis, focusing on future energy scenarios, by Marcel Fiebrandt from Thyssengas on the 25th of February at 10:00.
The EU's Green Deal and the German Federal Climate Protection Act, adapted in 2021, set the ambitious goal of achieving greenhouse gas neutrality by 2050 and 2045, respectively. The necessary transformation of the world's fourth-largest economy toward climate neutrality requires massive adjustments in all sectors and energy carriers as well as in the energy infrastructure. In the national and European hydrogen strategy, hydrogen in combination with renewable energy sources is assigned a central role for the transformation of the European energy system. This is the case as the properties of hydrogen are able to complement the increasing electrification of the sectors. For example, hydrogen can be stored in large underground caverns, transported in pipelines and used for power generation when renewable energy generation is insufficient, and it provides decarbonization opportunities in applications that cannot or are difficult to decarbonize through direct electrification. As a result, power-to-gas-systems, and especially electrolysers, will play a key role as the interface of electricity and gas infrastructure in the future integrated energy system to couple both energy carriers. In this talk, a brief overview of the future energy system scenarios and their implications on the industrial, mobility, and heating sector will be given. Afterwards, the options of producing hydrogen will be discussed and which requirements they have to fulfil to be integrated effectively and economically in the future energy system. Due to their critical role at the interface of the power and gas grid, special attention is paid to the technical requirements based on the properties and restrictions of the energy grid.
- Details
Awards
Heraeus Dissertation Award of the Faculty of Physics and Astronomy at RUB for Dr. Katharina Grosse (project B7)
The generation of plasmas in liquids is important for applications such as electrolysis, water purification or medicine, but also opens up a number of very fundamental questions. These plasmas are generated by short voltage pulses in the range of many kilovolts and a few nanoseconds in duration applied to a tungsten tip submerged in water. There is a lively debate around understanding the ignition of these plasmas, as electron multiplication during plasma ignition is postulated to occur either within small nanovoids, small fractures in the water, or as an electron avalanche in the water itself. In both cases, field emission at interfaces or field ionization of water molecules plays a crucial role. Dr. Grosse studied the whole dynamics of these plasmas from ignition to afterglow using time-resolved emission spectroscopy and compared it with modeling of emission and fluid dynamics. It showed that the broad continuum is produced by blackbody radiation, with a temperature of 7000 K, exactly equal to that of boiling tungsten. Electron densities of several 1025 m-3 can be derived from the strong broadening of the Balmer lines of the hydrogen atoms. Furthermore, a strong self-absorption of light from the region of the plasma channel is observed while light from the running ionization front shows no self-absorption. From this it can be deduced that the plasma runs directly through water and is not formed within nanovoids. Thus, field emission and field ionization dominate. After this first plasma pulse, the high power density leads to the phase transition from water to water vapor and bubble formation within the first microsecond. The high pressure in the range of GPa causes an expansion of the cavitation bubble and the generation of a sound wave propagating in the liquid. This could be directly observed using shadowgraphs. In particular, the speed of sound reaches several 1000 meters per second, indicating the very high pressure at the beginning of the discharge. Based on this measurement, Dr. Grosse has very significantly extended the understanding of these plasmas.
- Details
Award
Two awards for SFB researchers at the Academic Celebration 2021


PhD Student David Steuer recieved an award for his exceptional work during his master degree at the Academic Celebration of the Ruhr-University Bochum on the 24th of November. His thesis „Comparative Investigation of Two-Dimensional Oxygen Distributions in Microplasmas by Optical Methods” has been chosen to be the best master thesis in 2020 from the Faculty of Physics and Astronomy.
Additionally, Dr. Marco Krewing received the GdF-Award for exceptional interdisciplinary work during his dissertation with the title “Impact of low-temperature plasmas on microorganisms and biomolecules”. We congratulate both award winners to this special honor.
Images: M.Sc. David Steuer, Projects A6, B2 (left) and Dr. Marco Krewing, Project B8 (right)
- Details
Conversion of substances
DFG approves second funding period of the CRC 1316
Plasmas for the Systems for material conversion are an important component in the utilization and storage of decentrally generated renewable energies. The Collaborative Research Center 1316 (CRC 1316) "Transient Atmospheric Pressure Plasmas - from Plasma to Liquids to Solids" is dedicated to combining atmospheric pressure plasmas with catalysis to develop the most flexible solutions possible for this material conversion. "They should be scalable, controllable and robust against external influences, such as impurities in the starting materials," explains Prof. Dr. Achim von Keudell, spokesman of the CRC.
The first funding period of the CRC 1316 was dedicated to the elucidation of transient phenomena in atmospheric pressure plasmas as well as interfacial processes at the surface of catalysts. Here, the focus was on three systems: the plasma-catalytic conversion of gases, the combination of plasmas with electrolysis at the interface between liquid and solid, and plasma-assisted biocatalysis, in which enzymes very selectively produce new molecules. The researchers were able to make great progress in these areas: For example, they achieved precise control of the formation of reactive particles in these plasmas. They were also able to gain a deeper understanding of the atomic and molecular surface processes in these systems.
In the second funding period, these findings will be brought together to make the best possible use of the interplay between a plasma with its reactive particles and a catalytically active surface. There are many further questions in this regard, since in traditional catalysis, for example, stable molecules are essentially reaction partners, whereas in plasma catalysis, reactive particles or highly excited species can accelerate or suppress a specific reaction path. On this basis, the first prototype plants for plasma catalysis, plasma electrolysis and plasma biocatalysis are to be developed.
In addition to the RUB as the host university, researchers from the University of Ulm, the Jülich Research Center and the Fritz Haber Institute in Berlin are involved in the CRC.
- Details
Conference
Japan Workshop
A workshop between CRC1316 and Japanese universities/research institutions will take part between November 29th and December 3rd, 2021. The organizers are Prof. Czarnetzki, Satoshi Hamaguchi, Jan Kuhfeld and two PhD students from Nagoya University. Further information can be found here.
Please note that the deadline is already October 27. Active participation is by invitation only, but passive participation is completely open. Participants must register in any case.
- Details
Insights into the SFB 1316
Virtual public 360° tour of the SFB 1316
Insights into the projects and laboratories, the opportunity to take a look at the various experiments and diagnostics and ask live questions about them - this opportunity is available to everyone on 27.10.2021 at 4 pm during a virtual 360° tour. The tour is aimed at the general public and thus offers not only researchers and students but also interested persons outside of university the opportunity to experience research interactively and get to know the projects better.
- To participate in the virtual tour, registration is requested at
This email address is being protected from spambots. You need JavaScript enabled to view it. .
- Details
DIAGNOSTICS
Plasmas as chemistry labs
The smaller a plasma, the larger the experimental setup needed to study it. It is worth the effort, because the reaction conditions found in cubic-millimetre-sized plasmas are very much unique. Even though plasmas at atmospheric pressure are often only a few cubic millimetres in size, they pack quite a punch. This is because special non-equilibrium states can be set up in them, which facilitate physical and chemical processes that are not possible in any other environment. The plasma thus becomes a special kind of laboratory, where atoms and molecules can be excited without their surroundings heating up. “Such excitations could theoretically also be generated in a gas, but to do so we would have to heat it to several thousand degrees Kelvin. As a result, the molecules would decompose,” explains Professor Uwe Czarnetzki, Head of the Chair of Plasma and Atomic Physics at the Faculty of Physics and Astronomy. For many years, he and his team have been developing methods to explore the processes inside plasmas and to characterise the plasmas. Plasmas boast a unique feature: electric fields can be used to supply energy to the electrons in the plasma; the electrons in turn interact with molecules such as nitrogen or carbon dioxide while transferring the energy to them. The molecules are excited, and this happens without the environment heating up in the process, as would be the case in a gas. The molecules that are excited to vibrate have a much higher reactivity than those in the ground state. Plasma can therefore change chemistry or even enable certain chemical processes in the first place. Consequently, plasma provides basic researchers with a unique opportunity to study the excitation of molecules and the associated chemistry beyond thermodynamic equilibrium. Uwe Czarnetzki is therefore primarily interested in the vibrational states of molecules in plasmas.
- Details
MGK Colloquium
Virtual MGK Colloquium
The scientific exchange among the CRC members and the group of Early Career Researcher occurred continuously during the three yearly project meetings and in the workshops organized by the CRC 1316 since it starting in 01/2018. However, it is very important that the ECR have also a platform to interact in a conference setting without the impact of their adviser to stimulate the discussions among the ECR. Instead, the CRC 1316 decided to organize an MGK Colloquium on its own by inviting the ECR from the CRC 1316 and from the SFB-TR 87. This meeting was organized by the ECRs J. Kuhfeld and P. Preissing in a virtual format on 21/04/2021. Prominent invited speakers at this event were Prof. A. Bogaerts (university Antwerp), Dr. S. Iseni (GREMI, Orléans) and Dr. T.L. Chng (LPP Paris). Beside presentations within a zoom meeting, virtual poster sessions were performed, enhancing the interaction between the ECR.
- Details
Honour of research of project A5
Project A5 on the Inside Front Cover of Plasma Processes and Polymers
The current issue (April 2021) of Plasma Processes and Polymers features work from project A5 of the CRC 1316 on the topic of "positive and negative streamer propagation in volume dielectric barrier discharges with planar and porous electrodes" on its inside front cover.
The scientists have found that the discharge characteristics of negative surface streamers differ significantly from those of positive surface streamers. While negative streamers develop along the dielectric surface, allowing them to propagate into much smaller dielectric pores, positive streamers floatingly develop above the dielectric.
- The article on the research findings and the Inside Front Cover are freely accessible online.